


CasNo: 524-38-9
MF: C8H5NO3
Appearance: yellow moist powder
|
Purification Methods |
N -Hydroxyphthalimide [524-38-9] M 163.1, m 230o, ~235o(dec), 237-240o, 7.0. Dissolve the imide in H2O by adding Et3N to form the salt and while hot, acidify, cool and pour into a large volume of H2O. Filter off the solid, wash it with H2O and dry it over P2O5 in a vacuum. [Nefken & Teser J Am Chem Soc 83 1263 1961, Fieser 1 485 1976, Nefkens et al. Recl Trav Chim, Pays-Bas 81 683 1962] The O-acetyl derivative has m 178-180o (from EtOH). [Beilstein 21/11 V 100.] |
|
Application |
N-Hydroxyphthalimide is used in the synthesis of a new class of antibacterials potent against macrolide resistant bacteria. Also used in the synthesis of pyrazolidines, isoxazolidines and tetrahydrooxazines. |
InChI:InChI=1/C8H7NO3/c10-6-5-3-1-2-4-8(5,12)7(11)9-6/h1-5,12H,(H,9,10,11)
The reactivity of the phthalimide N-oxyl...
A novel synthesis of fused perfluoroalky...
Nicotinic acid, also known as niacin, is...
The invention belongs to the technical f...
The invention discloses a synthesis meth...
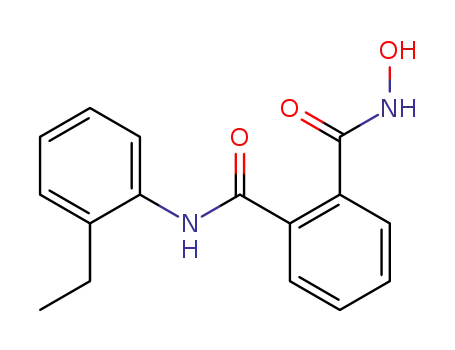
N-(2-ethyl-phenyl)-N'-hydroxy-phthalamide

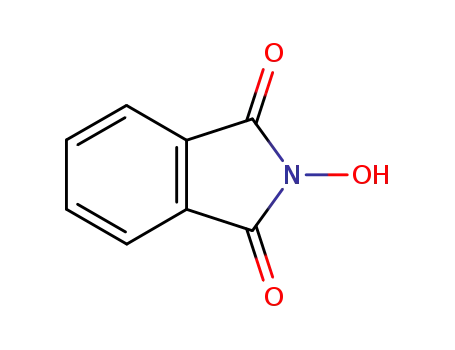
N-hydroxyphthalimide

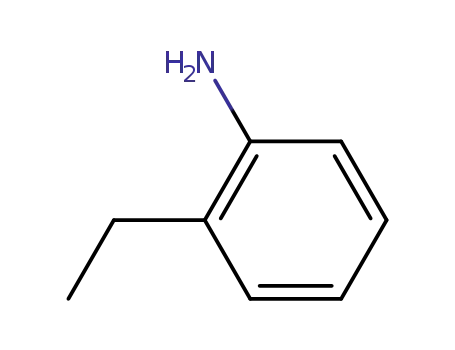
ortho-ethylaniline
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; acetonitrile; at 20 ℃;
|
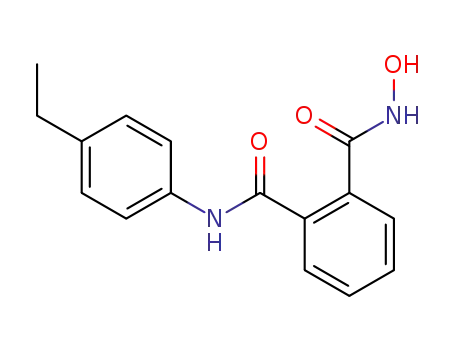
N-(4-ethyl-phenyl)-N'-hydroxy-phthalamide


N-hydroxyphthalimide

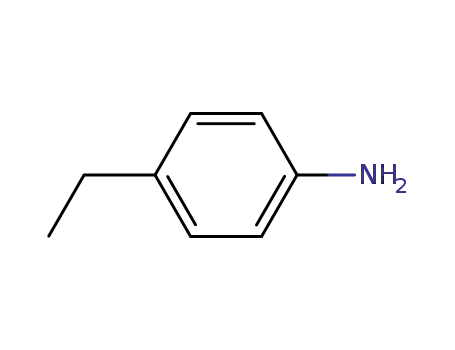
4-aminoethylbenzene
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; acetonitrile; at 20 ℃;
|
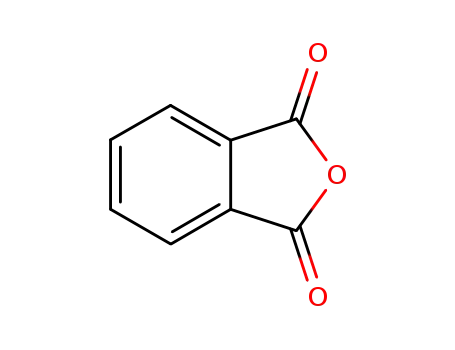
phthalic anhydride
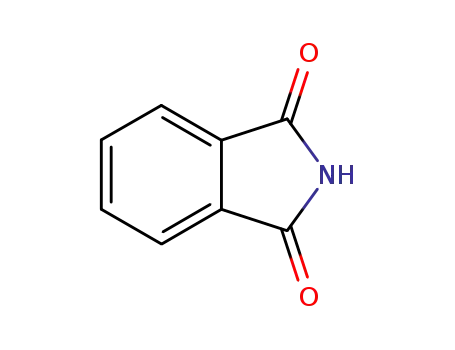
phthalimide
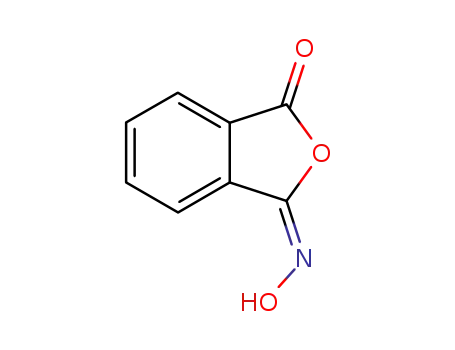
hydroxyimino-phthalide
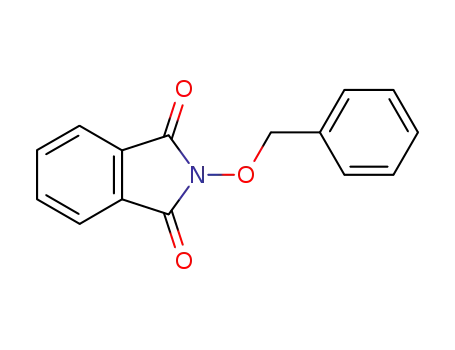
N-(benzyloxy)phthalimide
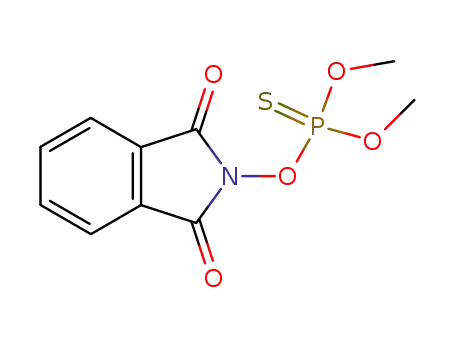
N-dimethoxythiophosphoryloxy-phthalimide
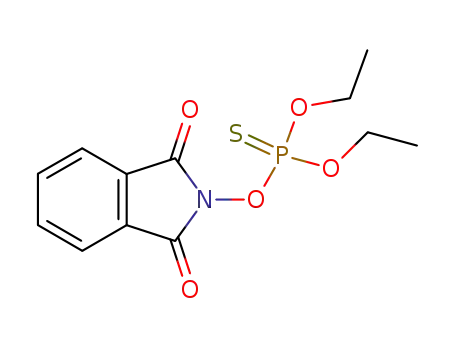
O,O-diethyl-phthalimido phosphorothioate
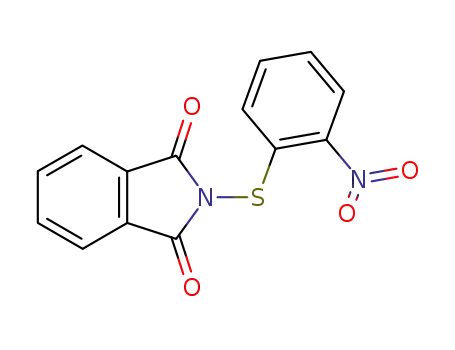
N-2-nitrophenylsulfenylphthalimide
N-(mesyloxy)phthalimide