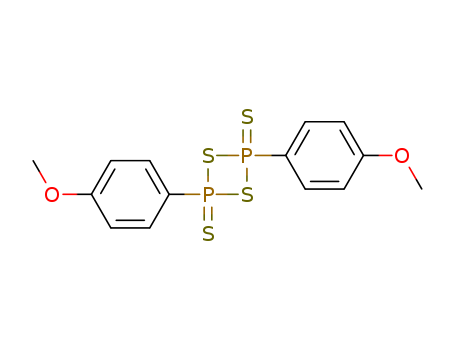


CasNo: 19172-47-5
MF: C14H14O2P2S4
Appearance: light yellow to beige powder
Cost-effective and readily available cat...
A sulfur-rich nickel complex has been sy...
Some 1,3-dithiadiphosphetane 2,4-disulfi...
Herein, the synthesis of three nickel(II...
![2-(4-Methoxy-phenyl)-[1,3,2]dithiaphospholane](/upload/2024/7/c92afdf2-28b2-4fec-ab8b-7b533be01d32.png)
2-(4-Methoxy-phenyl)-[1,3,2]dithiaphospholane

Lawessons reagent

ethene
| Conditions | Yield |
|---|---|
|
at 700 ℃; flash vacuum pyrolysis;
|
80% |
methoxybenzene


Lawessons reagent
| Conditions | Yield |
|---|---|
|
With tetraphosphorus decasulfide; for 6h; Heating;
|
88% |
|
With tetraphosphorus decasulfide; for 2h; Reflux;
|
82% |
|
With tetraphosphorus decasulfide; at 150 ℃; for 2h;
|
79.2% |
|
With tetraphosphorus decasulfide;
|
54.1% |
|
With phosphorus; sulfur; at 155 - 158 ℃; for 5.5h;
|
53.2% |
|
With tetraphosphorus decasulfide;
|
|
|
With tetraphosphorus decasulfide;
|
|
|
With tetraphosphorus decasulfide; at 150 ℃;
|
|
|
With tetraphosphorus decasulfide; for 2h; Heating;
|
|
|
With tetraphosphorus decasulfide; Heating;
|
|
|
With phosphorous (V) sulfide;
|
|
|
With phosphorous (V) sulfide;
|
|
|
With tetraphosphorus decasulfide; at 160 ℃; Inert atmosphere;
|
|
|
With tetraphosphorus decasulfide; Reflux;
|
|
|
With tetraphosphorus decasulfide; for 6h; Schlenk technique;
|
|
|
With phosphorus pentoxide; In neat (no solvent); at 160 ℃; Reflux;
|

methoxybenzene
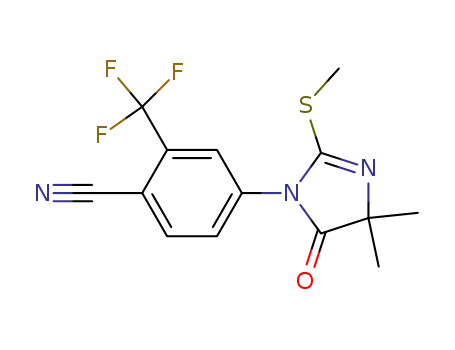
4-(4,5-dihydro-4,4-dimethyl-2-methylthio-5-oxo-1H-imidazol-1-yl) 2-trifluoromethyl-benzonitrile
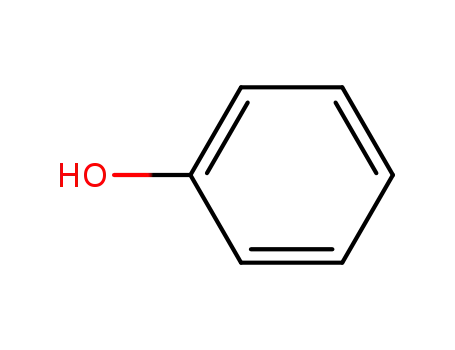
phenol
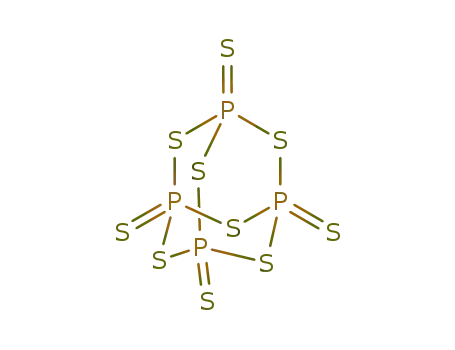
tetraphosphorus decasulfide
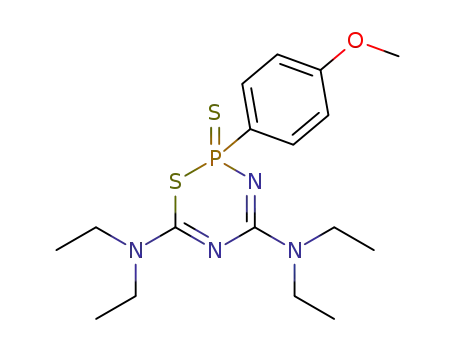
tetra-N-ethyl-2-(4-methoxy-phenyl)-2-thioxo-2H-2λ5-[1,3,5,2]thiadiazaphosphinine-4,6-diamine
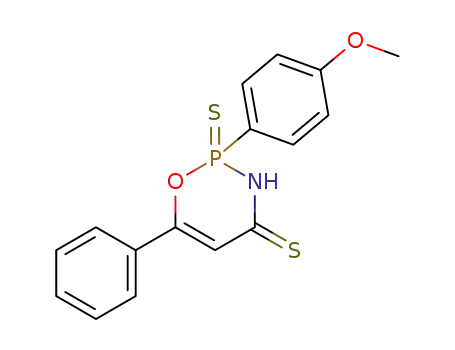
2-(4-methoxy-phenyl)-6-phenyl-2-thioxo-2,3-dihydro-2λ5-[1,3,2]oxazaphosphinine-4-thione
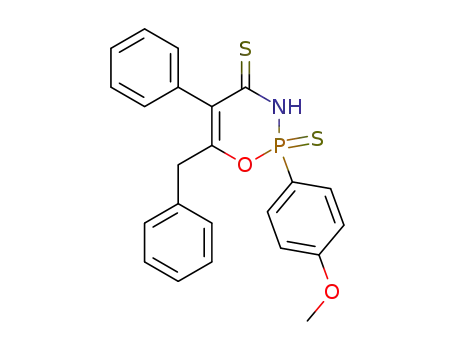
6-benzyl-2-(4-methoxy-phenyl)-5-phenyl-2-thioxo-2,3-dihydro-2λ5-[1,3,2]oxazaphosphinine-4-thione
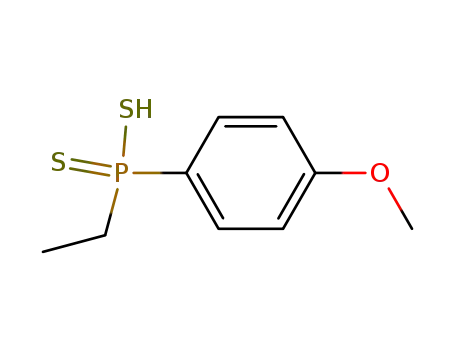
p-Methoxy-phenyl-aethyl-dithiophosphinsaeure