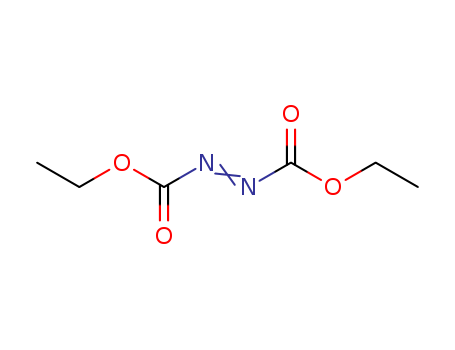


CasNo: 1972-28-7
MF: C6H10N2O4
Appearance: Clear orange to orange-red liquid
|
Chemical Description |
Diethyl azodicarboxylate is a reagent used in organic synthesis to introduce azo groups. |
|
Purification Methods |
Dissolve DEAD in toluene, wash it with 10% NaHCO3 till neutral (may require several washes if too much hydrolysis had occurred: check IR for OH bands), then wash with H2O (2x), dry over Na2SO4, filter, evaporate the toluene and distil it through a short Vigreux column (p 11) at as high a vacuum as possible. The main portion boils at 107-111o/15mm. Since it is likely to explode, use an oil bath for heating the still and all operations should be carried -out behind an adequate shield. [Rabjohn Org Synth Coll Vol III 375 1955, see Kauer Org Synth Coll Vol IV 412 1963]. [Beilstein 3 III 233.] It is commercially available as a 40% solution in toluene. This reagent is useful in the Mitsunobu reaction [Mitsunobu Synthesis 1 1981, Gennari et al. J Am Chem Soc 108 6394 1986, Evans et al. J Am Chem Soc 108 6394 1986, Hughes Org React 42 335 1992, Dodge et al. Org Synth 73 110 1996, Hughes Org Prep Proc Int 28 127 1996, Ferguson & Marcelle J Am Chem Soc 128 4576 2006; see also di-tert-butyl azodicarboxylate above and DIAD below]. § A polystyrene supported DEAD version is commercially available with a loading of ~1.2mmol/g. |
|
General Description |
DOT Special Approval Submission is Under Review. Availability is Estimated as March 2012. |
InChI:InChI=1/C6H10N2O4/c1-5(9)11-3-7-8-4-12-6(2)10/h3-4H2,1-2H3/b8-7+
-
-
A method has been developed for the synt...
Deconvolution of the role of off-cycle s...
-
PROBLEM TO BE SOLVED: To provide a metho...
PROBLEM TO BE SOLVED: To provide a metho...
PROBLEM TO BE SOLVED: To provide a metho...
The usefulness of flavin-based aerial ph...

4,7-Dimethyl-15-oxo-5,6-diphenyl-11,12-diazapentacyclo<8.2.2.14,7.02,9.03,8>pentadeca-5,13-dien-11,12-dicarbonsaeure-diethylester

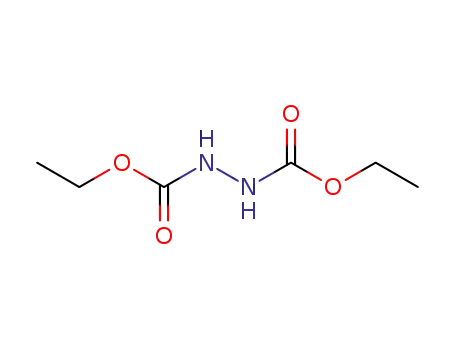
diethyl hydrazodicarboxylate

![methyl 3',6'-dimethyl[1,1*:2',1-terphenyl]-4'-carboxylate](/upload/2024/7/e23fe75b-ddce-40e1-99a2-09f83f085444.png)
methyl 3',6'-dimethyl[1,1*:2',1-terphenyl]-4'-carboxylate


4,9-Dimethyl-6,7-diphenyl-5,8-dioxa-13,14-diazahexacyclo<10.2.2.02,11.03,10.04,6.07,9>hexadec-15-en-13,14-dicarbonsaeure-diethylester

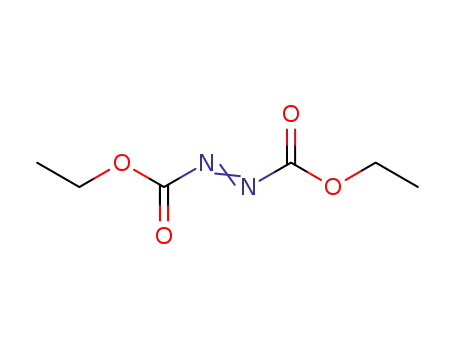
diethylazodicarboxylate
| Conditions | Yield |
|---|---|
|
In diethyl ether; acetone; at -25 - -20 ℃; for 6h; Irradiation;
|
46% 7% |

4,7-Dimethyl-15-oxo-5,6-diphenyl-11,12-diazapentacyclo<8.2.2.14,7.02,9.03,8>pentadeca-5,13-dien-11,12-dicarbonsaeure-diethylester


diethyl hydrazodicarboxylate

![methyl 3',6'-dimethyl[1,1*:2',1-terphenyl]-4'-carboxylate](/upload/2024/7/e23fe75b-ddce-40e1-99a2-09f83f085444.png)
methyl 3',6'-dimethyl[1,1*:2',1-terphenyl]-4'-carboxylate


4,9-Dimethyl-6,7-diphenyl-5,8-dioxa-13,14-diazahexacyclo<10.2.2.02,11.03,10.04,6.07,9>hexadec-15-en-13,14-dicarbonsaeure-diethylester


diethylazodicarboxylate

benzene
| Conditions | Yield |
|---|---|
|
In diethyl ether; acetone; at -25 - -20 ℃; for 6h; Product distribution; Irradiation;
|
46% 7% |
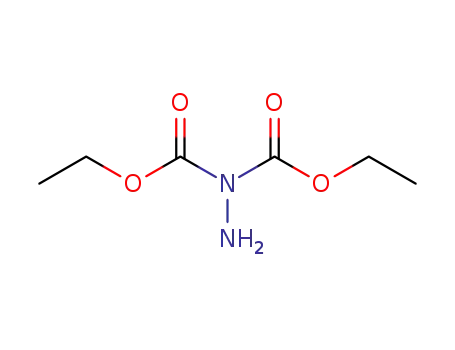
diethyl hydrazinedicarboxylate

diethyl hydrazodicarboxylate

benzene
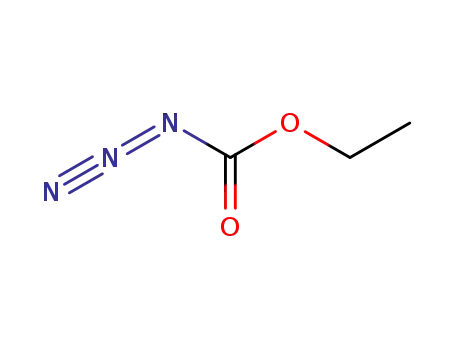
ethyl azidocarbonate
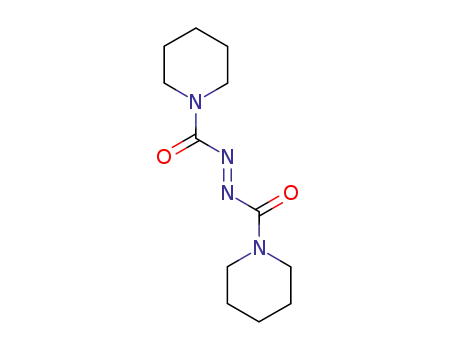
1,1'-(Azodicarbonyl)dipiperidin
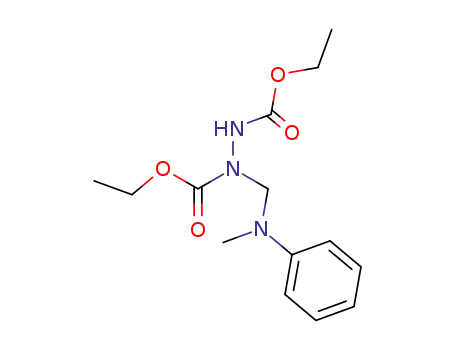
(N-methyl-anilinomethyl)-hydrazine-N,N'-dicarboxylic acid diethyl ester
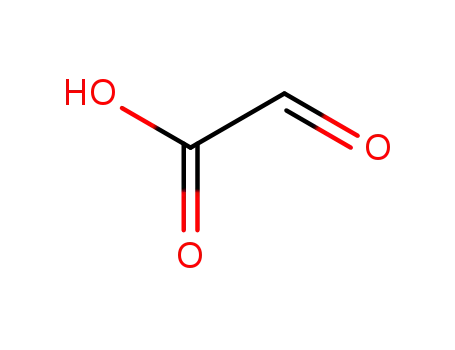
Glyoxilic acid
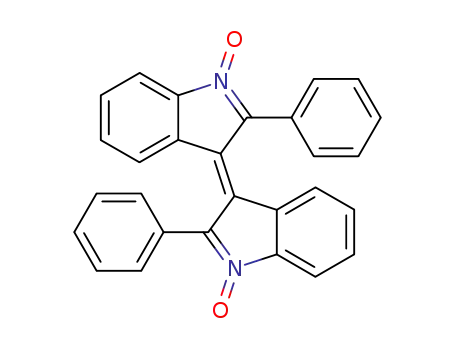
2,2'-diphenyl-[3,3']biindolylidene 1,1'-dioxide